TLDR
- Mira Pharmaceuticals stock soared 70% after-hours on Wednesday after preclinical results showed Mira-55 outperformed morphine in pain relief.
- The oral drug fully normalized pain and reduced inflammation in animal models, while morphine only partially reduced swelling.
- MIRA targets the $70 billion non-opioid pain market with plans to file an IND application for human trials.
- Retail sentiment turned “extremely bullish” with Stocktwits message volume jumping 7,000% as investors called it a potential blockbuster.
- The stock closed at $1.32 before surging to $2.23 in extended trading, with year-to-date gains now at 16%.
Mira Pharmaceuticals stock exploded 70% in after-hours trading Wednesday following breakthrough preclinical data. The company announced its oral drug candidate Mira-55 beat injected morphine in treating chronic inflammatory pain.
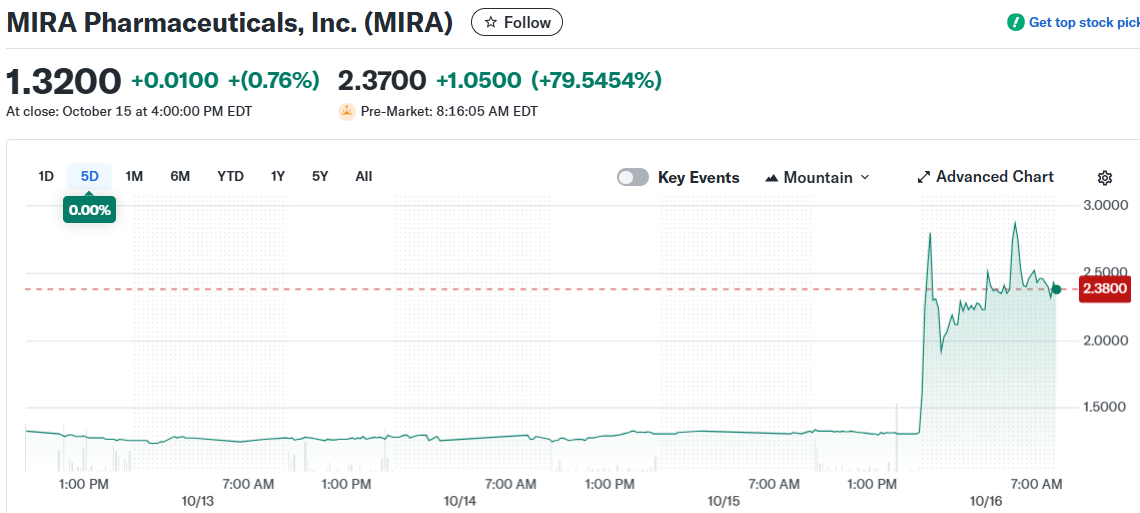
Shares closed regular trading at $1.32, up slightly for the day. The stock jumped to $2.23 in extended hours after the announcement.
The study used animal models to test Mira-55 against morphine. Researchers injected formalin into rat paws, a standard pain research method.
Mira-55 restored pain thresholds to baseline levels. The drug also prevented the swelling and inflammation that comes with this type of pain.
Morphine only partially reduced swelling through indirect central nervous system effects. The opioid couldn’t match Mira-55’s dual action.
How Mira-55 Works
The company said this was the first study to directly measure both inflammation and pain in the program. Mira-55 works through CB2 receptor mechanisms.
These receptors are part of the endocannabinoid system. They handle anti-inflammatory and pain-relief pathways without causing a high.
The drug is a non-psychotropic marijuana analog. It avoids THC’s psychoactive effects by targeting CB2 instead of CB1 receptors.
CEO Erez Aminov said Mira-55 delivers morphine-level relief without addiction or sedation. The company is targeting the $70 billion non-opioid pain market.
Mira plans to file an Investigational New Drug application with the FDA. The filing would allow the company to begin human trials for chronic inflammatory pain.
Pipeline Expansion
Mira is also developing Ketamir-2 for neuropathic pain. The ketamine analog received FDA IND clearance for U.S. trials earlier this year.
Phase 1 results in September showed the drug was safe with no serious side effects. The company expects to start Phase 2 trials in late 2025.
Mira recently acquired SKNY Pharmaceuticals. The deal added SKNY-1, a compound for obesity and nicotine addiction.
The acquisition brought $5 million in assets to the balance sheet. SKNY-1 caused 30% weight reduction in animal models.
The company reports third-quarter earnings on November 11. Mira posted a Q2 net loss of $0.09 per share with no revenue.





