TLDR
- Omeros Corporation shares rocketed 149% to $10.19 following a $2.1 billion licensing agreement with Novo Nordisk.
- The deal provides Omeros with $340 million in upfront payments for exclusive global rights to zaltenibart, a MASP-3 inhibitor.
- Trading volume hit 77.9 million shares versus the typical 1.2 million daily average.
- Omeros had only $28.7 million cash remaining as of June after spending $58.9 million in early 2025.
- The company awaits an FDA decision on its lead drug narsoplimab by December 26, 2025.
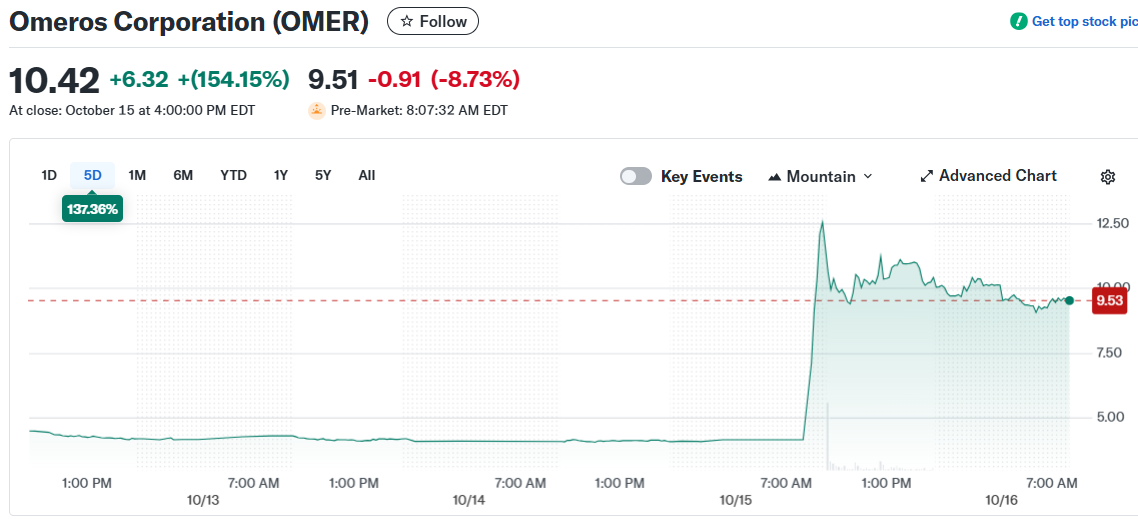
Omeros Corporation stock soared 149% on October 15, closing at $10.19. The previous day’s close was $4.10.
The catalyst was a licensing deal with Novo Nordisk worth up to $2.1 billion. The agreement covers zaltenibart, an experimental drug targeting rare blood and kidney disorders.
Novo Nordisk gets exclusive worldwide development and commercialization rights. The Danish pharmaceutical giant will pay $340 million upfront and near-term.
Shares opened at $11.53 and reached an intraday high of $12.77. The low was $4.33.
Volume exploded to 77.9 million shares compared to the normal 1.2 million. The stock trades on the Nasdaq Global Select Market. OMER has traded between $0.92 and $12.87 over the past year.
Payment Structure and Milestones
The total deal value could reach $2.1 billion through milestone payments. These depend on clinical development progress and commercial performance.
Omeros will also collect royalties if zaltenibart reaches market. The drug was previously known as OMS906.
Omeros management hinted at this deal during August earnings. They said zaltenibart negotiations were the most advanced among several potential asset sales.
The company’s market cap stood below $700 million at midday October 15. That’s less than half the total potential deal value.
Financial Situation and Pipeline
Omeros ended June with $28.7 million in cash and short-term investments. The company spent $58.9 million during the first six months of 2025.
The Novo Nordisk payment provides critical funding. It will cover debt payments and operating expenses for over a year.
Omeros generates no product revenue currently. The business runs at a loss.
Zaltenibart isn’t Omeros’ lead candidate. That’s narsoplimab, a MASP-2 inhibitor under FDA review.
Narsoplimab FDA Review Status
Narsoplimab targets stem cell transplant-associated thrombotic microangiopathy, or HSCT-TMA. The FDA rejected the initial application in 2021.
The agency requested additional data about treatment effectiveness. Omeros submitted new information last December.
The FDA originally scheduled a decision for September 25, 2025. That date was pushed to December 26, 2025 in August.
The 2021 rejection likely requested a randomized controlled trial. Omeros instead compared 28 treated patients against similar untreated patients.
Narsoplimab failed a separate pivotal trial in 2023. That study tested the drug for kidney damage in a different autoimmune condition.
Omeros now has capital to sustain operations while awaiting the narsoplimab decision. The FDA ruling by year-end will determine the fate of the company’s most advanced program.





