TLDR
- Plus Therapeutics (PSTV) stock surged 60% after UnitedHealthcare approved national coverage for CNSide® cancer test
- Agreement covers over 51 million patients nationwide, effective September 15, 2025
- H.C. Wainwright maintains Buy rating with $3.00 price target following insurance breakthrough
- CNSide® test shows 92% sensitivity and 95% specificity in detecting cancer cells
- Over 11,000 tests performed at 120+ cancer institutions since 2020
Plus Therapeutics stock exploded higher Thursday morning, gaining 60% in premarket trading after announcing a game-changing insurance agreement. The biotech company secured national coverage from UnitedHealthcare for its CNSide® cancer diagnostic test.
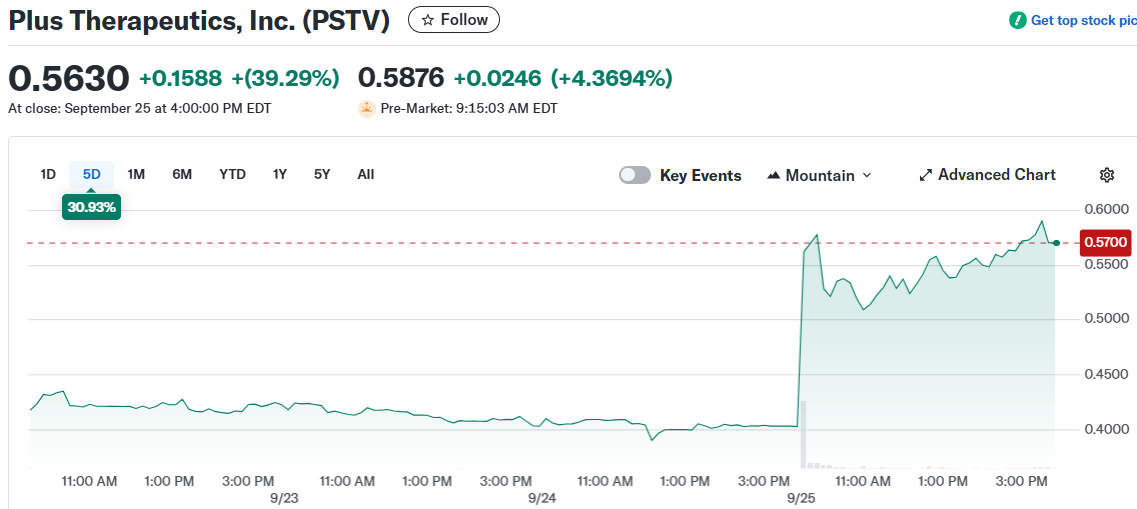
The deal, effective September 15, 2025, provides coverage for more than 51 million UnitedHealthcare members across the United States. This marks the largest insurance agreement in Plus Therapeutics’ history.
UnitedHealthcare will now cover the CNSide® Cerebrospinal Fluid Tumor Cell Enumeration test through the company’s wholly-owned subsidiary, CNSide Diagnostics. The test helps doctors diagnose and monitor patients with leptomeningeal metastases.
Wall Street responded positively to the news. H.C. Wainwright analyst Sean Lee maintained his Buy rating on PSTV stock with a $3.00 price target.
Strong Clinical Performance Data
The CNSide® test has demonstrated impressive accuracy in clinical settings. It delivers 92% sensitivity and 95% specificity when detecting cancer cells in cerebrospinal fluid.
Medical professionals have relied on the test to influence treatment decisions in 90% of cases. This high impact rate makes it a valuable diagnostic tool for oncologists treating complex cancer cases.
Since launching in 2020, healthcare providers have performed over 11,000 CNSide® tests. The diagnostic is now available at more than 120 cancer treatment centers throughout the United States.
Plus Therapeutics says the test outperforms standard diagnostic methods. Nine peer-reviewed publications and the FORESEE clinical trial support these claims.
The company recently received CLIA certification, opening doors for broader national distribution. Combined with UnitedHealthcare coverage, this creates a clear revenue growth pathway.
Analyst Optimism and Future Catalysts
Sean Lee expects additional positive developments at the upcoming Society for Neuro-Oncology annual meeting. Plus Therapeutics plans to present updates from its ReSPECT-LM study.
Early results from this study show promising response rates and favorable patient survival outcomes. These findings suggest strong potential for the company’s broader product pipeline.
The analyst’s $3.00 price target reflects confidence in Plus Therapeutics’ long-term revenue prospects. This valuation comes from a risk-adjusted analysis of the company’s innovative diagnostic products.
The UnitedHealthcare agreement provides access to a patient population previously unreachable through smaller regional insurance deals. This national coverage represents a major scaling opportunity for the CNSide® business.
Revenue from this agreement should appear in Plus Therapeutics’ fourth-quarter financial results. The September 15 effective date means the company is already generating income under the new coverage terms.
Plus Therapeutics now has established infrastructure to scale its cancer diagnostic testing nationwide through one of America’s largest health insurers.





