TLDR
- SPRB stock surged 1,378% to $130.40 after FDA Breakthrough Therapy Designation
- Tralesinidase alfa treats Sanfilippo syndrome type B, affecting one in 200,000 Americans
- Martin Shkreli disclosed long position with $500 price target
- Application for approval planned for Q1 2026
- Premarket trading showed additional 90% gain Tuesday
Spruce Biosciences stock posted one of the biggest single-day gains of 2025 on Monday. SPRB shares jumped 1,378% to close at $130.40.
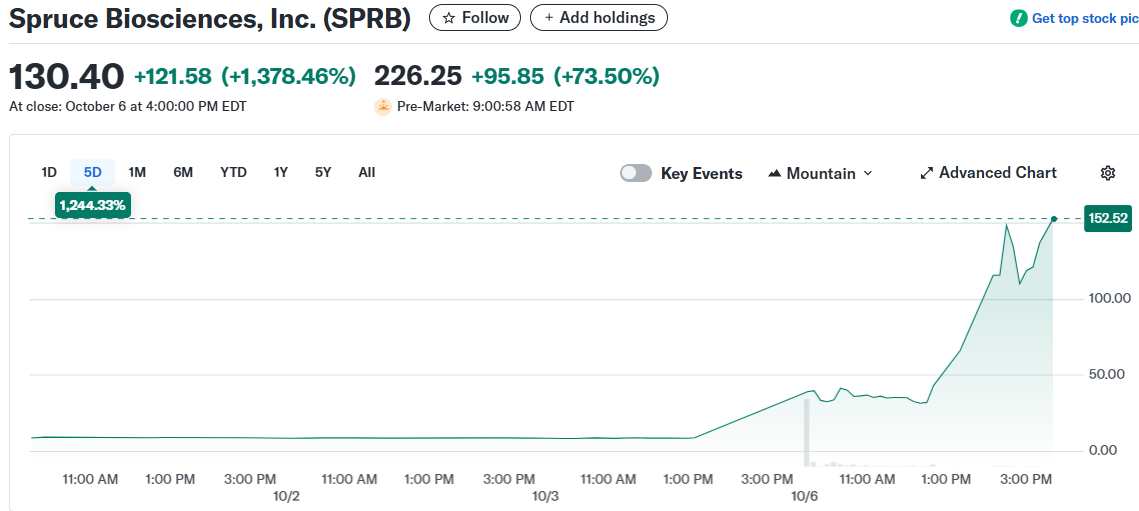
The catalyst was the FDA’s Breakthrough Therapy Designation for tralesinidase alfa. This enzyme replacement therapy targets Sanfilippo syndrome type B.
The rare genetic disorder affects approximately one in 200,000 people in the United States. It causes progressive neurological decline in children.
What Is Breakthrough Therapy Designation?
The FDA Breakthrough Therapy Designation accelerates drug development and review. Companies receive intensive guidance from regulators throughout the process.
This status is reserved for treatments addressing serious or life-threatening conditions. The therapy must show substantial improvement over existing options.
Tralesinidase alfa works by normalizing heparan sulfate levels in patients. Without treatment, Sanfilippo syndrome type B patients typically die by age 18.
The therapy could extend lifespans to normal ranges. It restores chemical imbalances that cause motor skill decline and cognitive issues.
Shkreli Weighs In With Price Target
The stock extended gains in Tuesday premarket trading, rising another 90%. Former pharmaceutical executive Martin Shkreli disclosed his position in the company.
Shkreli posted on social media that he expects the treatment will gain approval. He set a price target of $500 per share without providing a timeline.
“This drug restores the abnormally high levels of heparan sulfate to normal,” Shkreli wrote. He said patients may live normal lives with the treatment.
The investor served time for securities fraud. He recently revealed short positions in other stocks.
Company Plans 2026 Submission
CEO Javier Szwarcberg said the company is preparing its regulatory application. The biologics license application for MPS IIIB treatment will be submitted in Q1 2026.
Spruce Biosciences focuses on rare endocrine disorders. The company holds a market cap of $76.61 million.
Revenue stands at $1.3 million with an operating margin of -3,900.39%. The debt-to-equity ratio measures 0.14.
Liquidity ratios sit at 2.6 for both current and quick ratios. Institutional ownership is 4.85% while insiders hold 2.32%.
The stock carries a beta of 3.41, indicating high volatility. Analyst consensus rates the stock as a hold.
The price-to-sales ratio reached 233.93 following Monday’s rally. EPS is -85.91, reflecting ongoing operational losses.
The company’s pipeline includes tildacerfont in addition to tralesinidase alfa. Both target rare disease markets with limited treatment options.
The Q1 2026 application timeline represents the next major catalyst for SPRB stock.





